Abstract
Background:
The role of autologous stem cell transplantation (autoSCT) in non-high-risk acute myeloid leukemia (AML) is controversial. It remains a frequent consolidation alternative for AML in first complete remission (CR1) in Europe and Asia, especially for intermediate risk disease, but has fallen out of favor over allogeneic transplantation (alloSCT) in the United States (U.S.). Donor availability, however, affects feasibility of alloSCT in ethnic and racial minorities that remain disproportionately underrepresented in donor registries. We explored the survival experience in our predominantly minority non-high-risk AML patients treated with autoSCT in comparison to those treated with chemotherapy alone, the current standard of care in the U.S. for favorable-risk AML as well as for intermediate-risk disease when alloSCT is not available.
Methods:
All adult patients diagnosed with AML between the years 1997 - 2015 were identified within the Montefiore Medical System in the Bronx, NY. For the purpose of our analysis we only included AML cases with favorable or intermediate cytogenetic risk that received induction with aggressive chemotherapy, achieved CR1 and underwent consolidation with high-dose chemotherapy alone or chemotherapy followed by autoSCT. We used descriptive statistics to compare baseline characteristics of patients treated with one of the two consolidation modalities. We used the Kaplan Meier survivor function to compare overall survival (OS) and relapse-free survival (RFS) between the two groups and multivariable Cox Proportional Hazard models to adjust for key covariates.
Results:
We identified 149 newly diagnosed adult AML cases with favorable (n=14) or intermediate-risk (n=135) cytogenetic risk. Among the 126 patients treated with aggressive chemotherapy, 96 (76%) achieved CR1 after induction chemotherapy, among which 10 experienced early relapse, 3 were lost to follow up and 83 proceeded to consolidation with either high-dose chemotherapy alone (n=58) or chemotherapy followed by autoSCT (n=25).
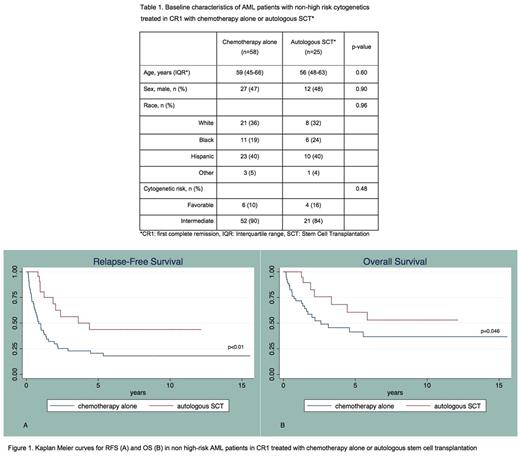
The cohort was predominantly non-white (n=50/83, 60%). The median age was 57 years. Distribution of age, sex, race and cytogenetic-risk was similar between the two treatment groups (table 1). Median follow up was 17.7 months.
Median OS was significantly longer for patients consolidated with autoSCT compared to those treated with chemotherapy alone (1.9 vs. 1.2 years; p=0.046). Recipients of autologous transplantation also had longer RFS compared to those consolidated with chemotherapy alone (1.6 vs. 0.6 years; p<0.01) (Figure 1). No treatment-related deaths were observed in the autoSCT group.
In multivariable analysis adjusted for age, sex, race and cytogenetic risk, patients who underwent consolidation with autoSCT had a significantly better OS (HR 0.28; 95%CI 0.11 - 0.72; p<0.01) and RFS (HR 0.20; 95%CI 0.09 - 0.45; p<0.001) compared to those treated with chemotherapy alone.
Conclusions:
In this minority-rich non-high-risk AML cohort, we showed that consolidation with autologous transplantation provided OS and RFS benefit compared to chemotherapy alone. Survival rates in our multiethnic cohort were comparable to those historically reported in predominantly white AML cohorts. Autologous transplantation remains a beneficial treatment option in non-high risk AML in first remission and is a particularly valuable therapeutic strategy for ethnic/racial minorities when an allogeneic donor is not available. Integration of minimal residual disease assessment may help refine the selection of those more likely to benefit from this approach.
Steidl: Novartis: Research Funding; Aileron Therapeutics: Consultancy, Research Funding; GlaxoSmithKline: Research Funding; Celgene: Consultancy; Bayer Healthcare: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal